MCSGP with AutoPeak® Technology
Summary
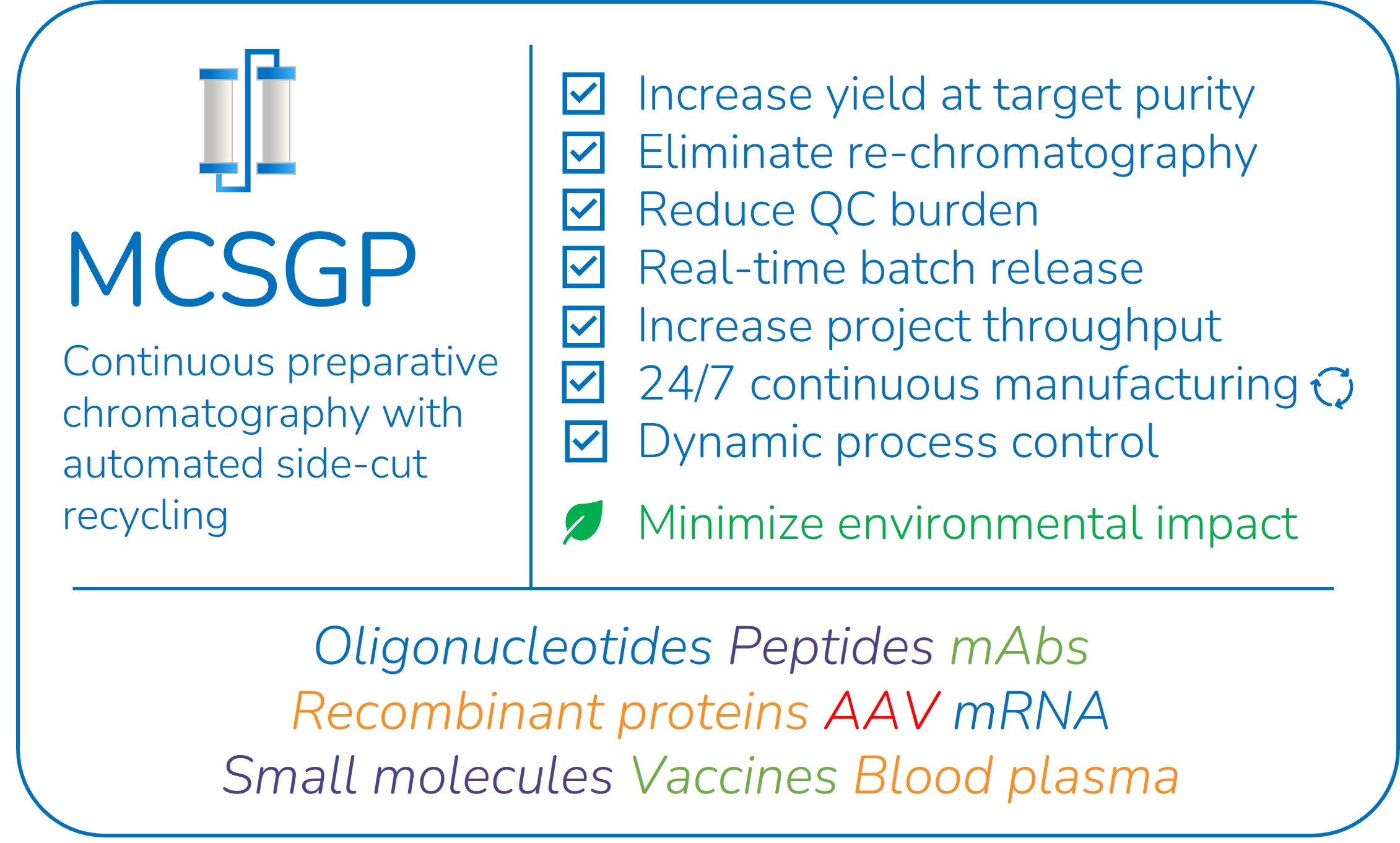
MCSGP avoids the manufacturing bottlenecks of standard preparative chromatography
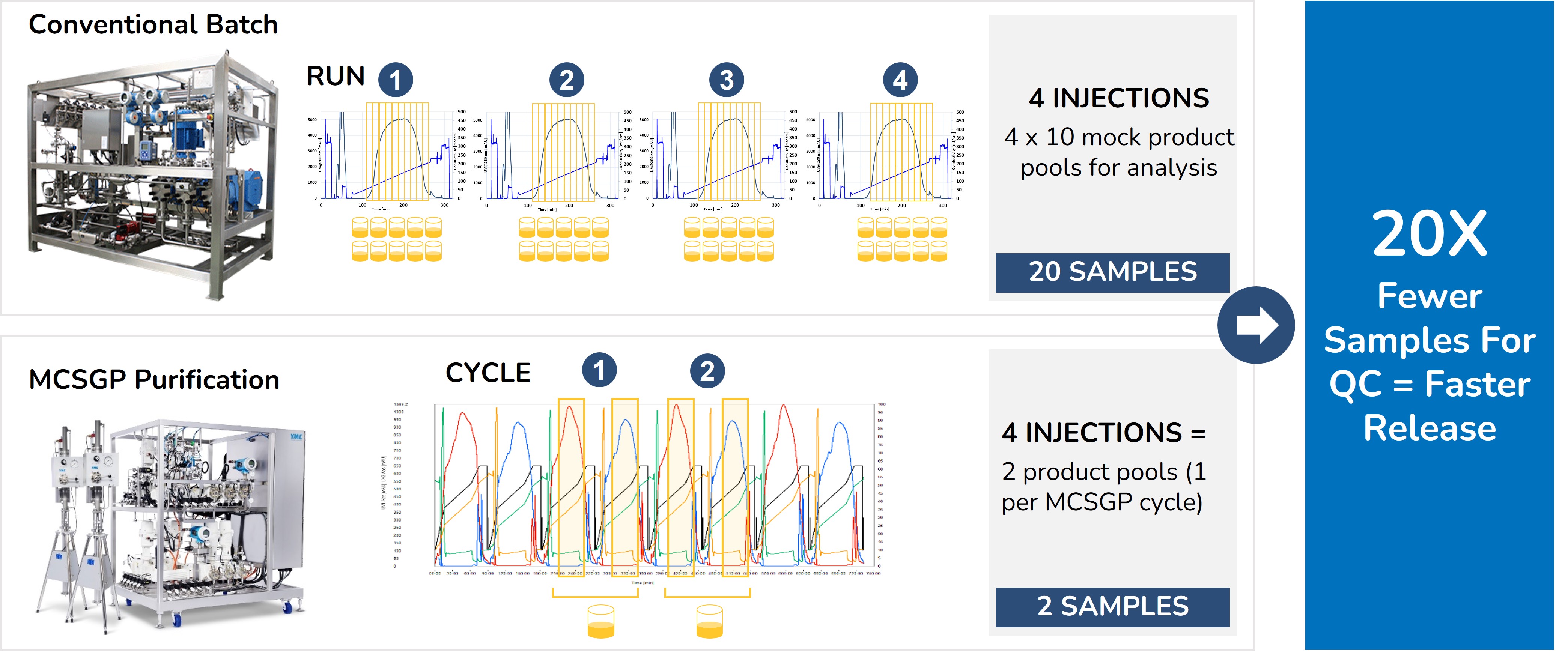
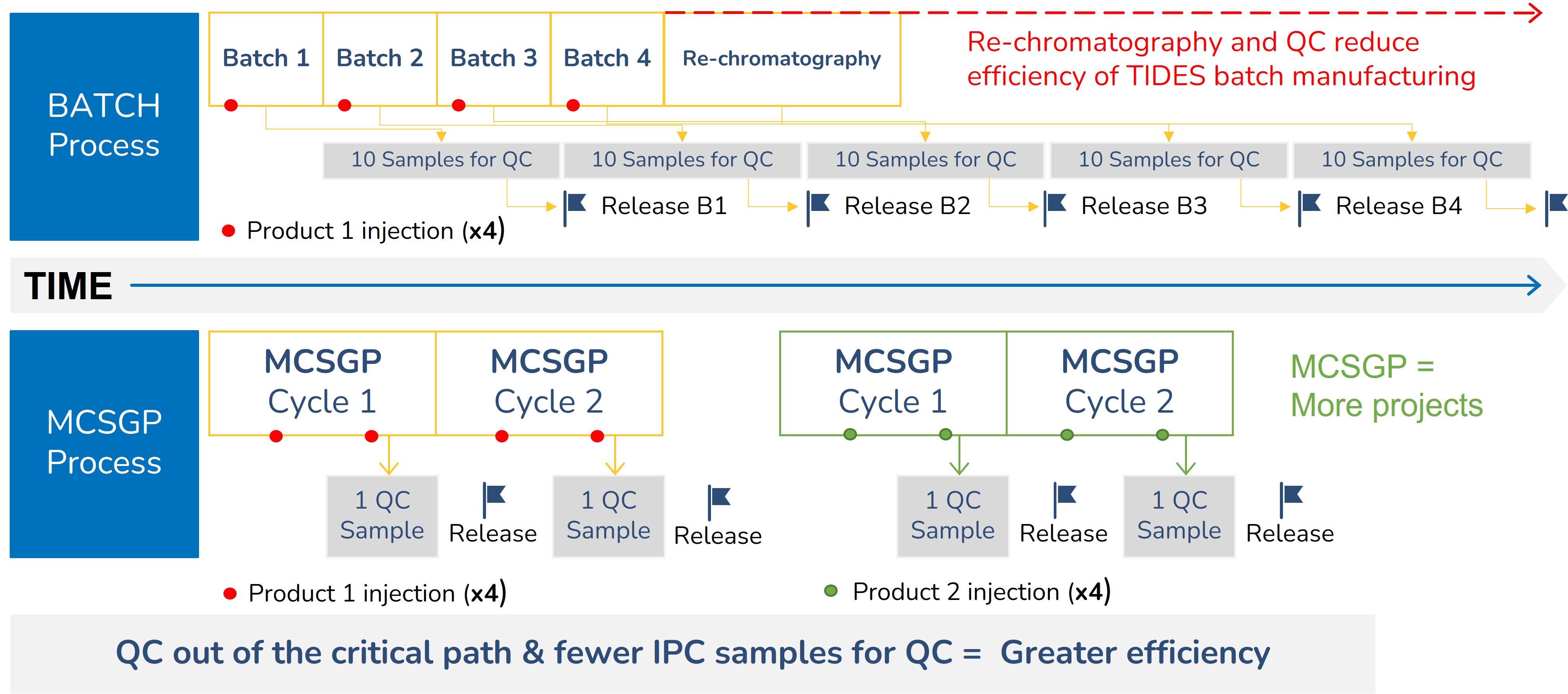
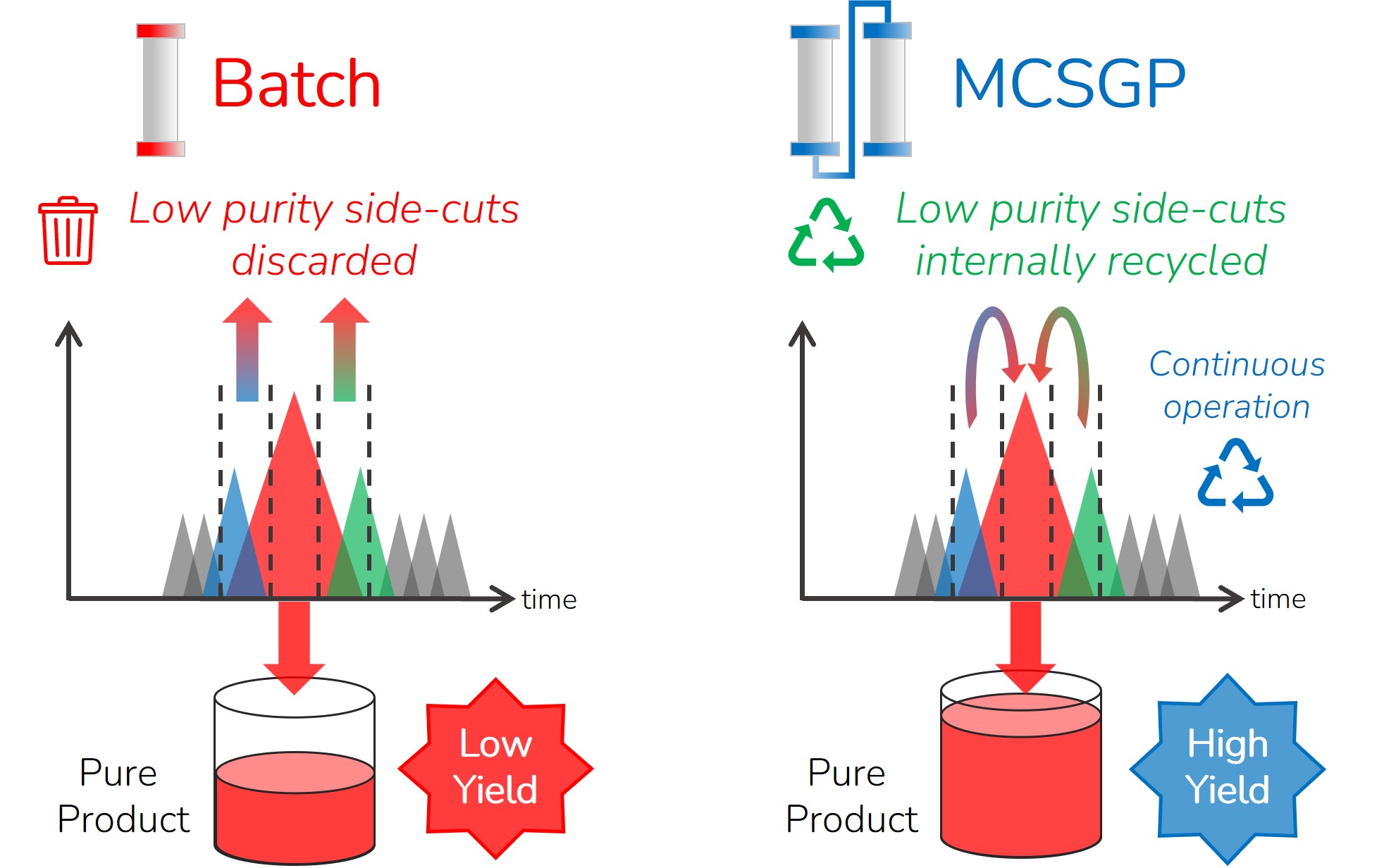
MCSGP (Multi-column Counter-current Solvent Gradient Purification) is a superior alternative to the preparative batch purification which is normally used in manufacturing of peptides and oligonucleotides. MCSGP is a continuous purification process integrating automated side-cut recycling & in-line dilution between two identical columns (See the MCSGP process principle). The automated side-cut recycling greatly increases yields and eliminates extra re-chromatography runs often done in batch manufacturing to improve yield. MCSGP also reduces the QC burden by eliminating the need for fractionation & mock pool QC analysis for every batch run. Instead, only the product pool is analyzed for quality. Altogether, MCSGP improves manufacturing efficiency, process economics and environmental sustainability.
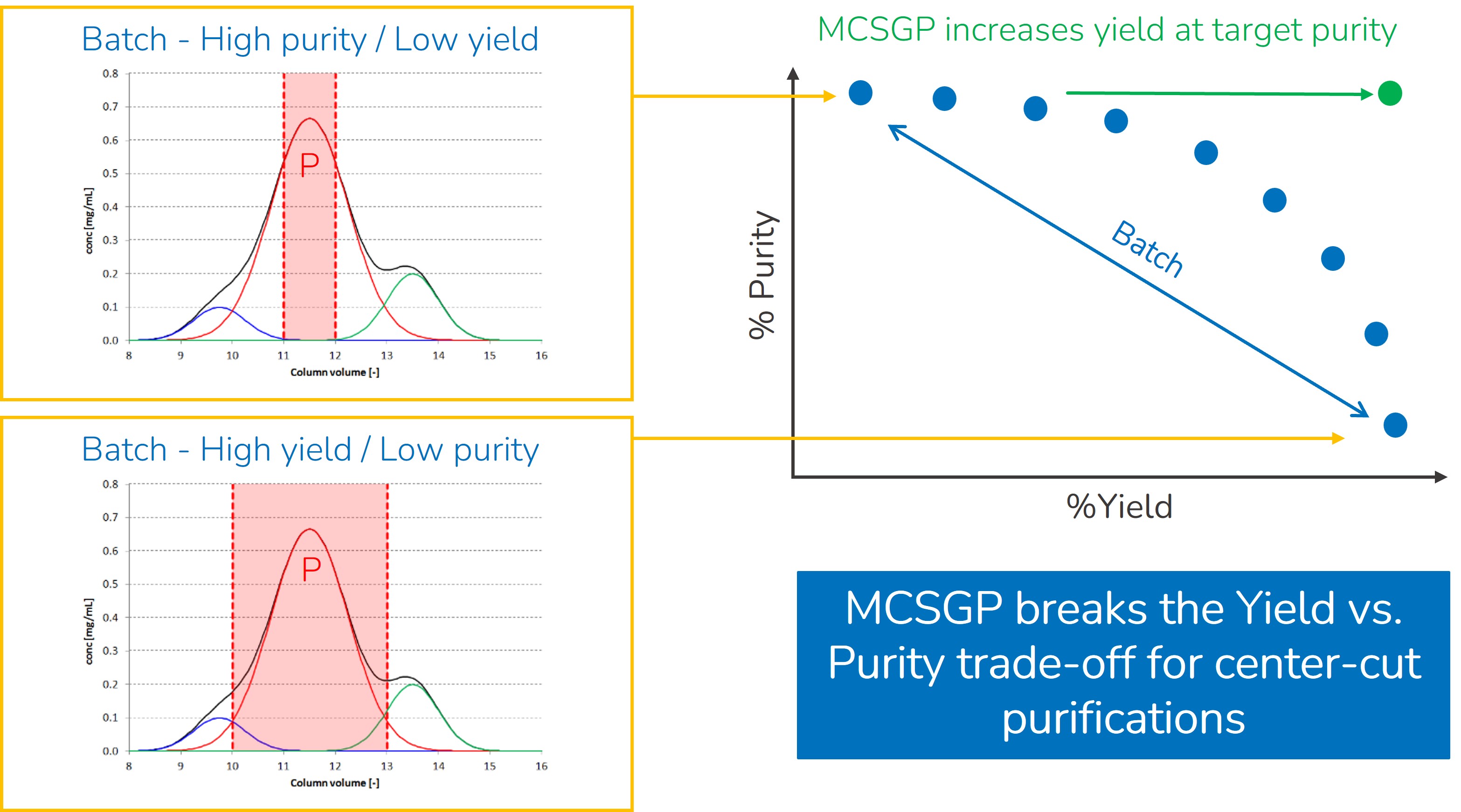
MCSGP increases process yields without sacrificing purity or productivity
MCSGP helps achieve environmental sustainability goals through process intensification
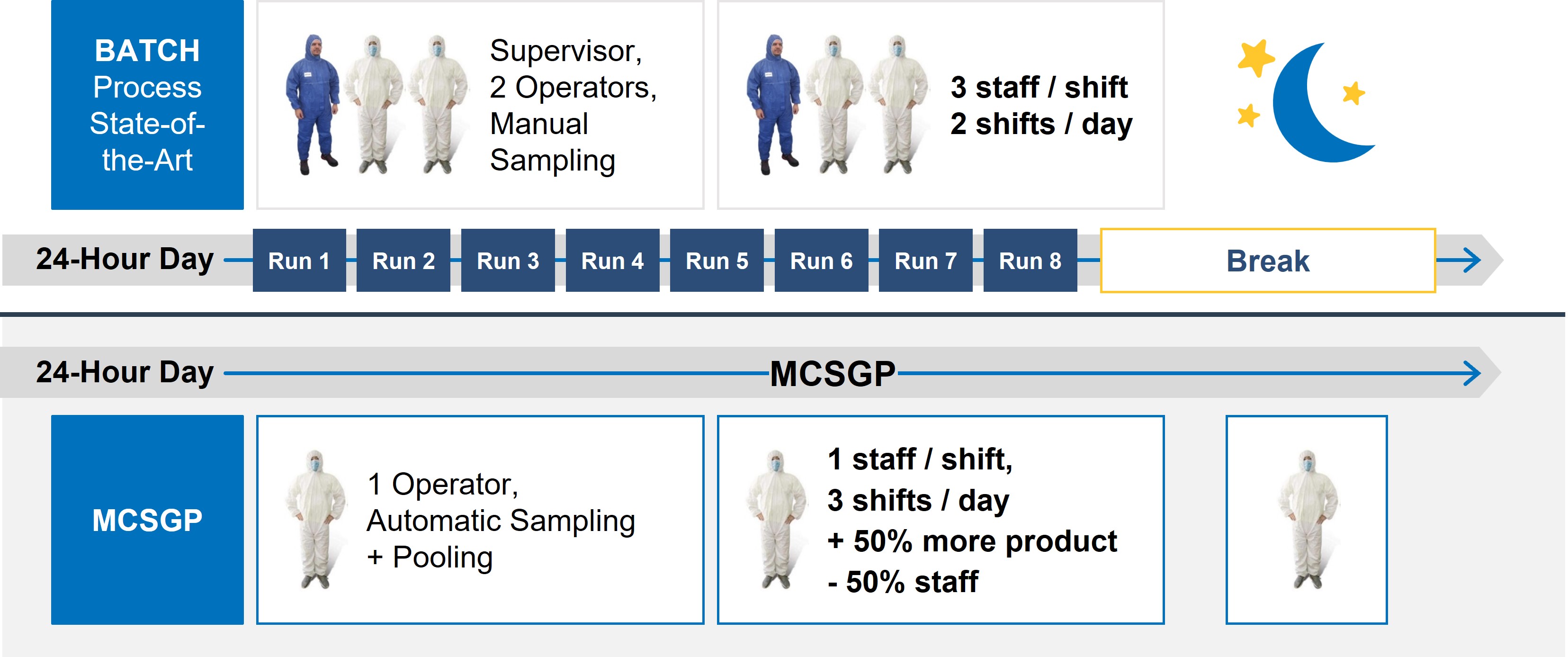
MCSGP can help minimize environmental impacts because it is both leaner and more intensive than preparative batch chromatography. MCSGP boosts process yields, eliminates inefficient re-chromatography runs, and greatly reduces analytical QC burden in a GMP environment. Together these advantages allow for a significant improvement in overall process economics and environmental benefits due to a significant reduction in material and labor requirements. Firstly, less feedstock is required for the same product output; secondly, no side-cut re-chromatography leads to savings on custom storage bags, dedicated storage space & analytical QC support; Thirdly, far fewer in-process control (IPC) samples are generated in MCSGP, lowering the QC burden and speeding up batch release times.
MCSGP eliminates re-chromatography steps with automated side-cut recycling
MCSGP Resources:
MCSGP Webinars
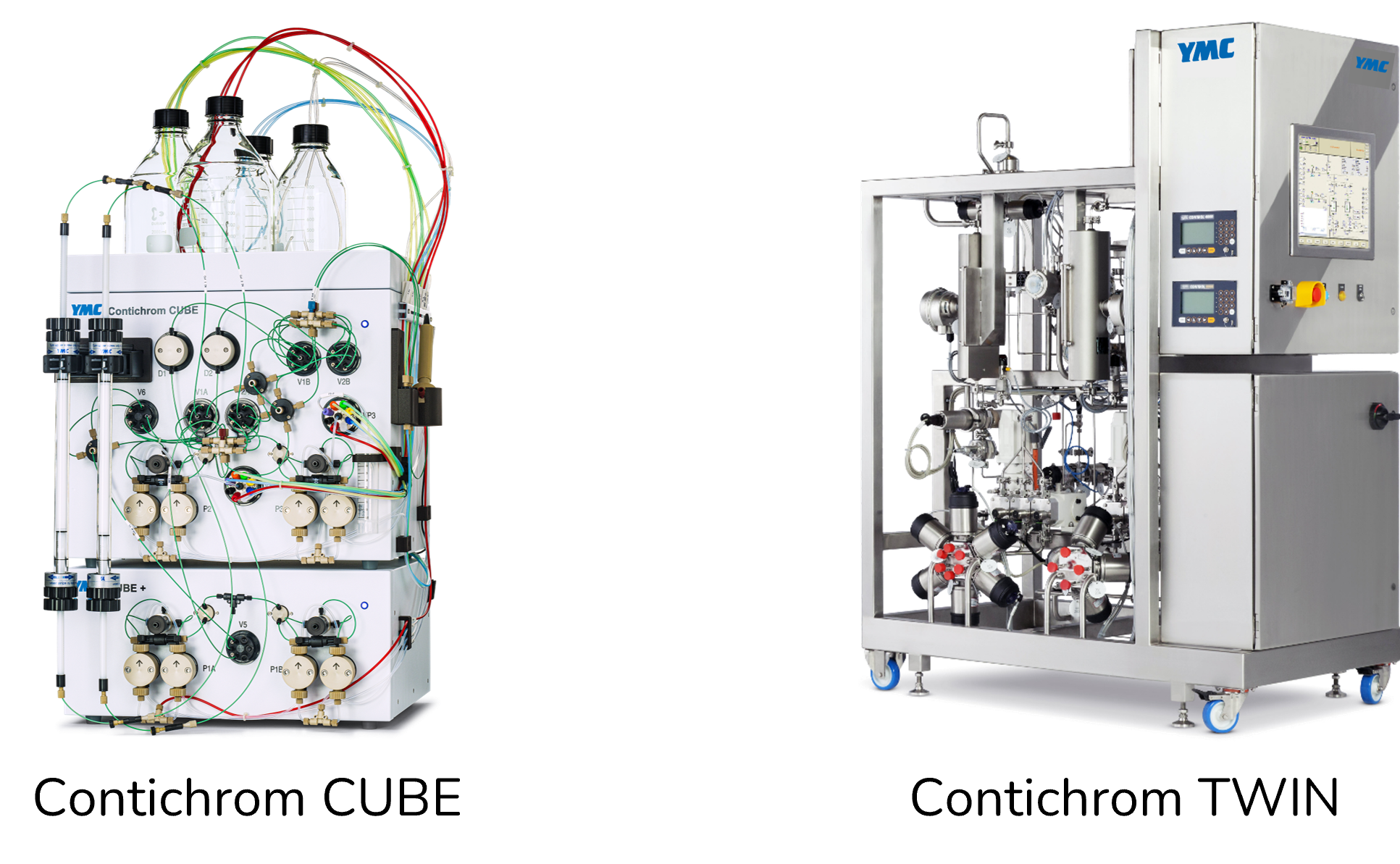
Introduction to MCSGP, Contichrom CUBE, and Contichrom TWIN
MCSGP Application Notes (pdf)
-
MCSGP Process Development – Part 1: Gradient Development
-
MCSGP Process Development – Part 2: MCSGP Optimization
-
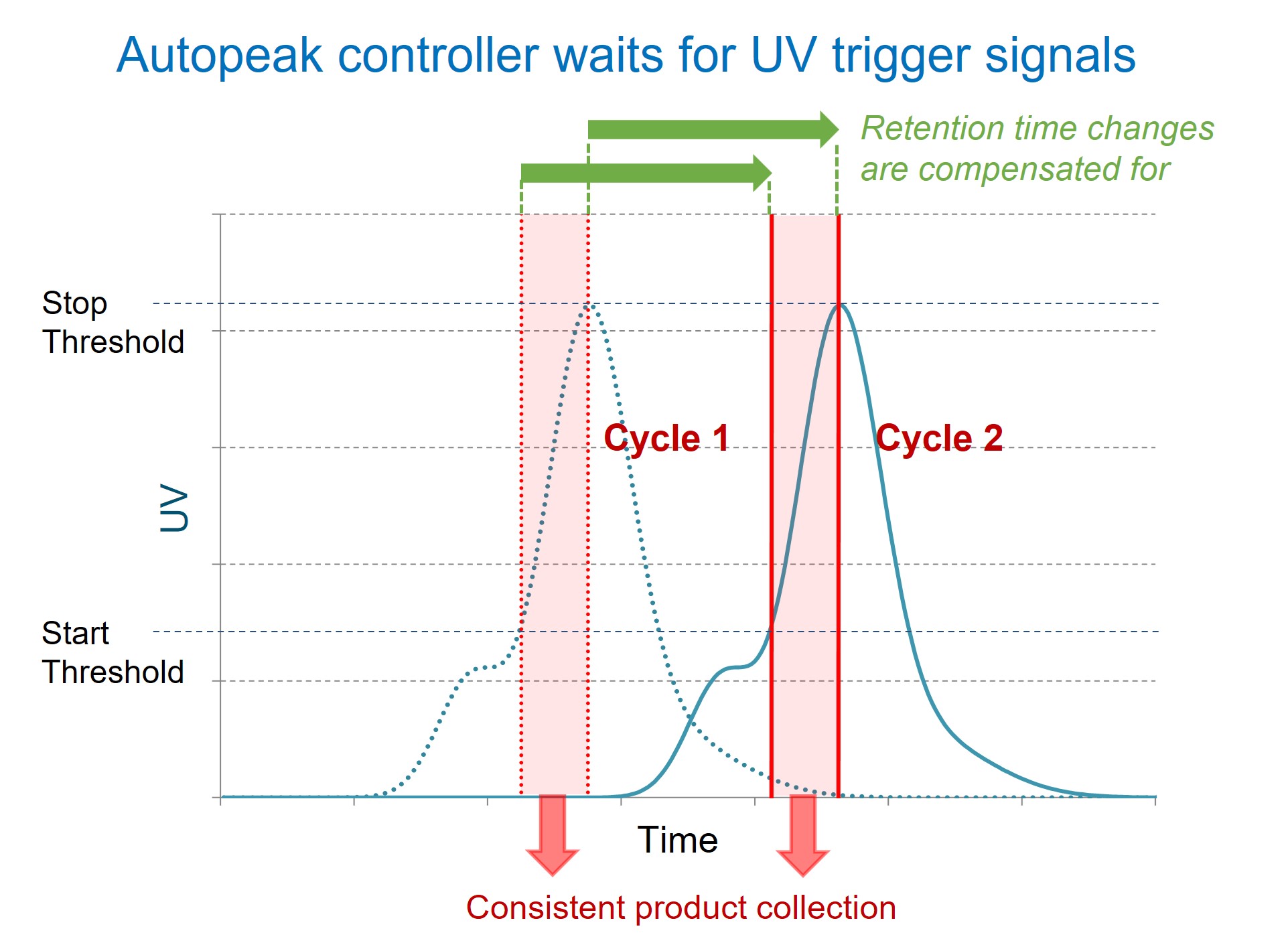
Dynamic Process Control (Autopeak) for Countercurrent Polish Processes (MCSGP)
-
Purification of a Therapeutic Oligonucleotide Using Twin-Column Chromatography (MCSGP)
-
Highly pure cannabidiol (CBD) by twin-column chromatography (MCSGP)
MCSGP Selected Publications
-
Purification of a GalNAc-cluster-conjugated oligonucleotide by reversed-phase twin-column continuous chromatography, Richard Weldon, Jörg Lill, Martin Olbrich, Pascal Schmidt, Thomas Müller-Späth, Journal of Chromatography A, Volume 1663, 2022, 462734. (open access)
-
Process Intensification for the Purification of Peptidomimetics: The Case of Icatibant through Multicolumn Countercurrent Solvent Gradient Purification (MCSGP). De Luca, C., Felletti, S., Bozza, D., Lievore, G., Morbidelli, M., Sponchioni, M., Cavazzini, A. 2021. Ind . Closely. Chem. Res. 2021, 60, 18, 6826-6834
-
Oligonucleotides: Current Trends and Innovative Applications in the Synthesis, Characterization, and Purification. Catani, M., De, C., Medeiros Garcia Alcântara, J., Manfredini, N., Perrone, D., Marchesi, E., Weldon, R., Müller‐Späth, T., Cavazzini, A., Morbidelli , M., Sponchioni, M., Biotechnol. J. 2020, 1900226.
-
Experimental design of a twin-column countercurrent gradient purification process. Steinebach F, Ulmer N, Decker L, Aumann L, Morbidelli M Journal of Chromatography A 2017, 1492, 26
-
Enabling high purities and yields in therapeutic peptide purification using multicolumn countercurrent solvent gradient. T. Müller-Späth, G. Ströhlein, O. Lyngberg, D. Maclean, Chimica Oggi-Chemistry Today 2013, 31 (5), 56-61 . (open access)
-
Purifying Common Light-Chain Bispecific Antibodies: A Twin-Column, Countercurrent Chromatography Platform Process. Müller-Späth T, Ulmer N, Aumann L, Ströhlein G, Bavand M, Hendriks LJA, de Kruif J, Throsby M, Bakker ABH, BioProcess International 2013, 11(3), 36-45 . (open access)