CaptureSMB with AutomAb® Control
Summary
Introduction
Affinity capture chromatography is a crucial step for the efficient processing of large volumes of cell culture harvest. However, despite suboptimal process performance, single-column batch chromatography is still standard due to it's simplicity. The superior alternative to single-column batch processing is Periodic CounterCurrent (PCC) purification, which offers significant economic benefits. ChromaCon has developed an optimized twin-column PCC process called CaptureSMB, the most effective and least complex PCC process available to date. To facilitate CaptureSMB development, we offer the Contichrom CUBE, a specialized twin-column FPLC system with a CaptureSMB wizard for software aided method creation.
How does CaptureSMB work?
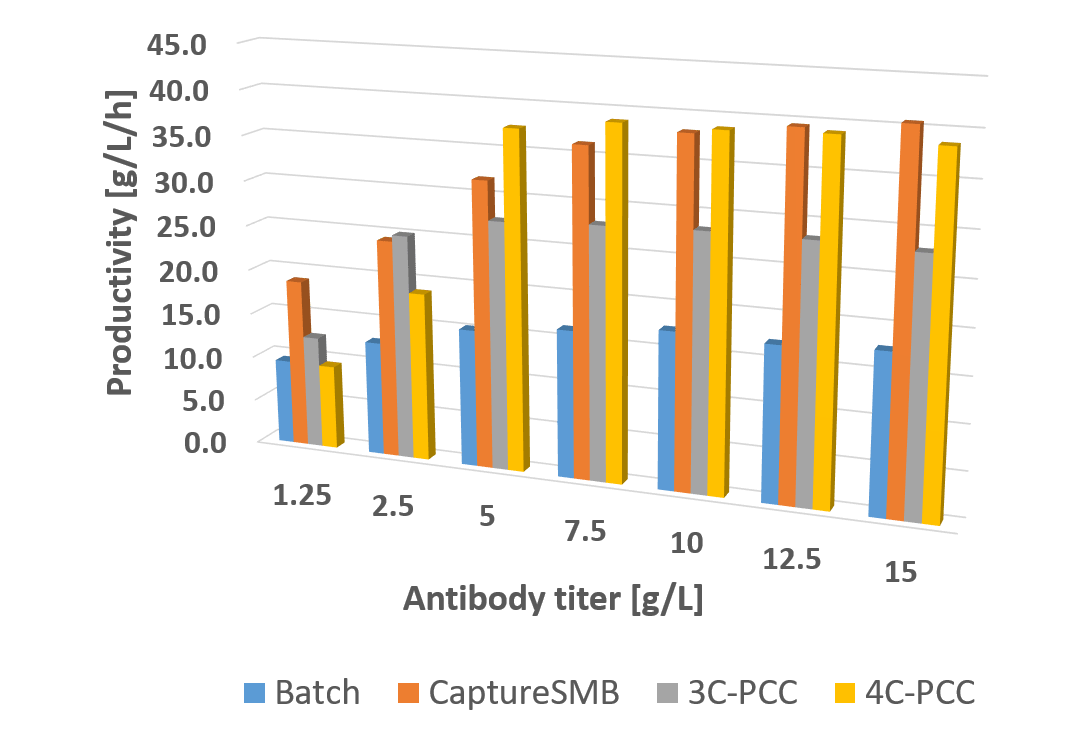
Unlike batch chromatography, which uses only 50-60% of resin material, CaptureSMB fully loads one column and captures product breakthrough on a second interconnected column, ensuring maximum product capture and efficient utilization of all available affinity resin (see process principle). This technology offers two-fold faster processing times and up to 50% savings in Protein A resin and buffer.
How does CaptureSMB compare to 3 or more column processes?
With only two columns, CaptureSMB has the least complex system configuration compared to multi-column systems, providing a de-risked operation while still benefiting from continuous processes. CaptureSMB has been implemented at an industrial scale on Contichrom TWIN GMP systems and has demonstrated superior performance in recent publications . Furthermore, a virus clearance study has shown that the CaptureSMB process has equivalent virus clearance compared to a batch process.
CaptureSMB Resources
Application Notes (pdf)
Links to Selected Publications
-
Scale-Up of Twin-Column Periodic Counter-Current Chromatography for mAb Purification. Angelo J, Pagano J, Müller-Späth T, Mihlbachler K, Chollangi S, Xu X, Ghose S, Li ZJ. BioProcess Int, 2018 Apr;16(4):28-37 (Open access)
-
Model assisted process characterization and validation for a continuous two-column protein A capture process
D Baur, J Angelo, S Chollangi, T Müller-Späth, X Xu, S Ghose, ZJ Li, M Morbidelli. Biotechnology & Bioengineering 2018, advance online publication . -
Continued insights into virus clearance validation across continuous capture chromatography. Angelo J, Potter K, Müller‐Späth T, Xu X, Li Z, Ghose S. Biotechnology & Bioengineering 2021, advance online publication .
-
Virus Clearance Validation across Continuous Capture Chromatography. Angelo J, Chollangi S, Müller‐Späth T, Jusyte S, Xu X, Ghose S, and Li Z. (2019). biotech. Bioeng. Accepted Author Manuscript .
-
Twin-column CaptureSMB: a novel cyclic process for protein A affinity chromatography
M Angarita, T Müller-Späth, D Baur, R Lievrouw, G Lissens, M Morbidelli, Journal of Chromatography A 2015, 1389, 85-98 . -
Affinity Capture of F (ab')2 Fragments: Using Twin-Column Countercurrent Chromatography
N Ulmer, T Müller-Späth, B Neunstoecklin, L Aumann, M Bavand, M Morbidelli, BioProcess International 2015, 13 (2), 22-29 (Open access)