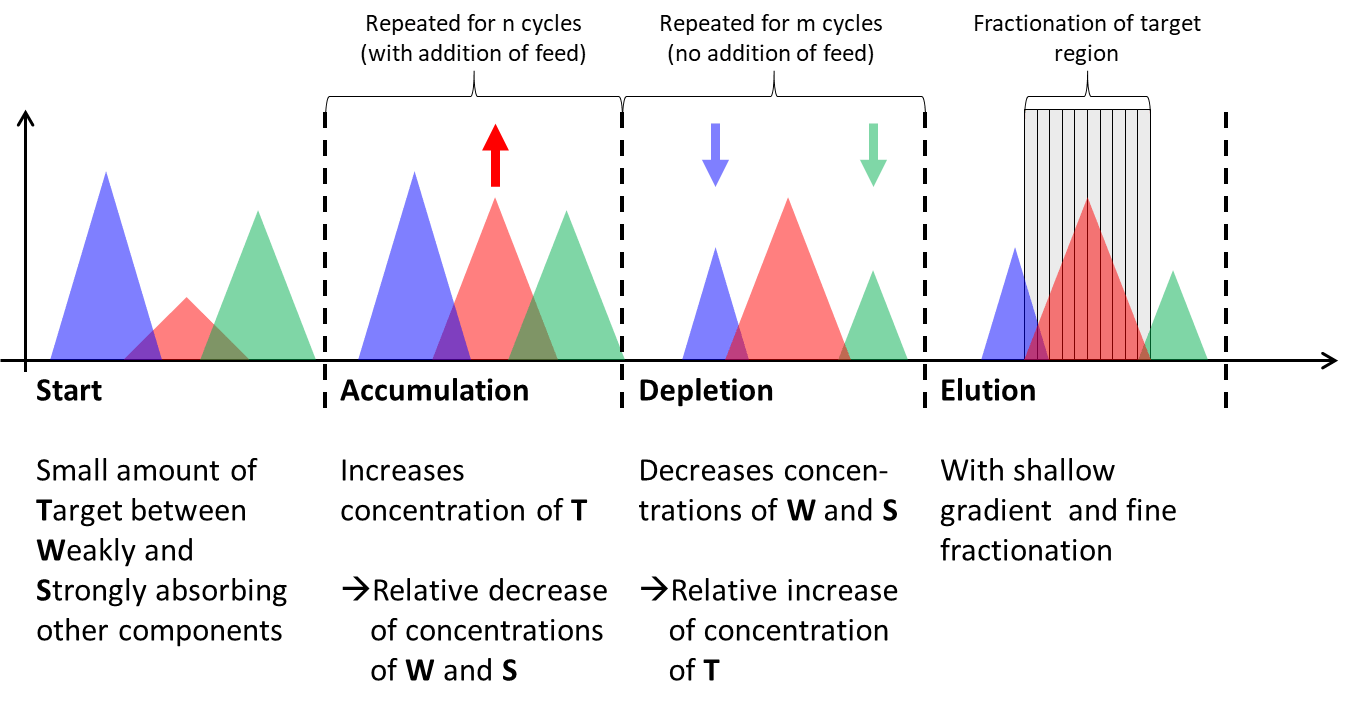
Impurity Isolation using N-Rich
Application example
Biosimilar interchangeable claims require comparison, of product-related impurities with the licensed reference product. Knowing product-related molecular variants requires isolating sufficient quantities of eg truncated, modified or aggregated forms for characterization. Without N-Rich, these investigations require tedious and repetitive purification procedures using analytical high resolution FPLC/HPLC with low loading capacity. N-Rich eliminates this bottleneck by combining the capabilities of preparative chromatography with the high resolution of a cyclic enrichment and polishing process.
The capabilities of the N-Rich technology are shown in an application note for isolating antibody isoforms or product-related impurities of a synthetic peptide. Isolation time was cut from more than 30 days, as required with conventional approaches, down to only 3 days, yielding quantities of 1.5 mg to 8 mg isolated proteins with purities of 75% to >90%.
Application areas of N-Rich
- Isolation of product-related impurities for
- Biosimilar characterization
- Active Pharmaceutical Ingredient (API) characterization
- Antibody-drug conjugate isoform characterization
- Studying isoform stability as a function of time and/or formulation
- R&D applications, including
- Identification of biomarkers and compounds in proteomics and metabolomics research
- Generation of ultra-pure compounds for
- protein crystallography
- reference materials